Ice and Clathrate Formation
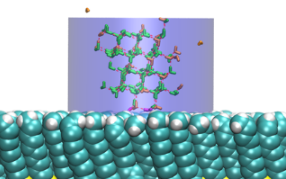
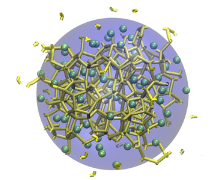
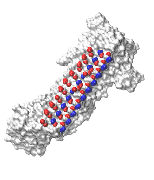
Ice formation is an important consideration in many contexts, such as the preservation of organs for transplantation, preventing the formation of clathrate hydrate plugs in natural gas pipelines, preserving food or biological matter, and understanding the function of anti-freeze proteins.
To achieve control over the formation of ice, it is crucial to understand its nucleation at a molecular level. Studies have shown that heterogenous nucleation of ice occurs much more readily than homogenous nucleation. That is, ice likes to form on interfaces. Thus, an understanding of ice nucleation in real-world contexts requires identifying the molecular-scale features of surfaces that inhibit or promote its growth.
We use and develop novel techniques to characterize the formation and growth of ice in order to gain a deep understanding of the relationships between the chemistry and topology of a surface and the resulting thermodynamics of ice formation on that surface.
- Relevant Publications
- AU Thosar, Y Shalom, I Braslavsky, R Drori and AJ Patel. The Accumulation of Antifreeze Proteins on Ice is Determined by Adsorption, ChemRxiv.
- SM Marks, Z Vicars, AU Thosar and AJ Patel. Characterizing Surface Ice-philicity Using Molecular Simulations and Enhanced Sampling, The Journal of Physical Chemistry B (2023).
- SM Marks and AJ Patel. Antifreeze Protein Hydration Waters: Unstructured Unless Bound to Ice, Proceedings of the National Academy of Sciences, 115, 8244-8246 (2018).
- Current People
- Zac Vicars
- Jeongmoon Choi
- Yusheng Cai